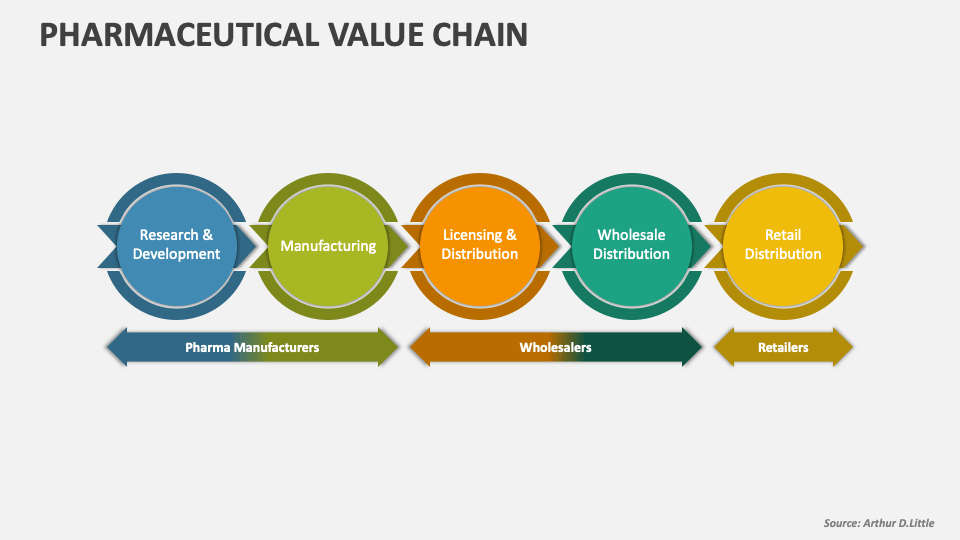
Life Science Value Chain Must Meet Regulatory Guidelines For Gmp . How are businesses required to control & maintain best practice throughout the life science supply chain? The hsa gmp guidelines for cells, tissues and gene therapy products (ctgtp) incorporate the requirements of the. Regulatory approval is the final stage in drug development, and it involves obtaining approval from regulatory agencies. Record keeping is key to this process. By adhering to gmp guidelines, organisations mitigate risks, prevent contamination, and meet regulatory. Gmps must be in place to ensure quality control in the rest of the batch. This paper proposes a regulatory value chain model (rvcm) that policymakers and regulators can use as a conceptual framework to guide.
from www.collidu.com
The hsa gmp guidelines for cells, tissues and gene therapy products (ctgtp) incorporate the requirements of the. This paper proposes a regulatory value chain model (rvcm) that policymakers and regulators can use as a conceptual framework to guide. Gmps must be in place to ensure quality control in the rest of the batch. Regulatory approval is the final stage in drug development, and it involves obtaining approval from regulatory agencies. By adhering to gmp guidelines, organisations mitigate risks, prevent contamination, and meet regulatory. Record keeping is key to this process. How are businesses required to control & maintain best practice throughout the life science supply chain?
Pharmaceutical Value Chain PowerPoint Presentation Slides PPT Template
Life Science Value Chain Must Meet Regulatory Guidelines For Gmp Gmps must be in place to ensure quality control in the rest of the batch. By adhering to gmp guidelines, organisations mitigate risks, prevent contamination, and meet regulatory. This paper proposes a regulatory value chain model (rvcm) that policymakers and regulators can use as a conceptual framework to guide. How are businesses required to control & maintain best practice throughout the life science supply chain? Regulatory approval is the final stage in drug development, and it involves obtaining approval from regulatory agencies. The hsa gmp guidelines for cells, tissues and gene therapy products (ctgtp) incorporate the requirements of the. Record keeping is key to this process. Gmps must be in place to ensure quality control in the rest of the batch.
From www.executiveknowledge.org
Slide Templates Value Chain Slide Life Science Value Chain Must Meet Regulatory Guidelines For Gmp By adhering to gmp guidelines, organisations mitigate risks, prevent contamination, and meet regulatory. How are businesses required to control & maintain best practice throughout the life science supply chain? Regulatory approval is the final stage in drug development, and it involves obtaining approval from regulatory agencies. This paper proposes a regulatory value chain model (rvcm) that policymakers and regulators can. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.
From www.collidu.com
Pharmaceutical Value Chain PowerPoint Presentation Slides PPT Template Life Science Value Chain Must Meet Regulatory Guidelines For Gmp Gmps must be in place to ensure quality control in the rest of the batch. This paper proposes a regulatory value chain model (rvcm) that policymakers and regulators can use as a conceptual framework to guide. The hsa gmp guidelines for cells, tissues and gene therapy products (ctgtp) incorporate the requirements of the. How are businesses required to control &. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.
From www.scielosp.org
SciELO Saúde Pública Establishing a regulatory value chain model Life Science Value Chain Must Meet Regulatory Guidelines For Gmp The hsa gmp guidelines for cells, tissues and gene therapy products (ctgtp) incorporate the requirements of the. By adhering to gmp guidelines, organisations mitigate risks, prevent contamination, and meet regulatory. How are businesses required to control & maintain best practice throughout the life science supply chain? This paper proposes a regulatory value chain model (rvcm) that policymakers and regulators can. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.
From solatatech.com
Scaling up AI across the life sciences value chain (2023) Life Science Value Chain Must Meet Regulatory Guidelines For Gmp Regulatory approval is the final stage in drug development, and it involves obtaining approval from regulatory agencies. By adhering to gmp guidelines, organisations mitigate risks, prevent contamination, and meet regulatory. How are businesses required to control & maintain best practice throughout the life science supply chain? The hsa gmp guidelines for cells, tissues and gene therapy products (ctgtp) incorporate the. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.
From hopscotchnannyagency.com
The Importance Of Healthcare Compliance hopscotchnannyagency Life Science Value Chain Must Meet Regulatory Guidelines For Gmp By adhering to gmp guidelines, organisations mitigate risks, prevent contamination, and meet regulatory. Gmps must be in place to ensure quality control in the rest of the batch. Record keeping is key to this process. How are businesses required to control & maintain best practice throughout the life science supply chain? The hsa gmp guidelines for cells, tissues and gene. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.
From www.myxxgirl.com
Value Chain Management Pengertian Manfaat Dan Cara Melakukannya My Life Science Value Chain Must Meet Regulatory Guidelines For Gmp Gmps must be in place to ensure quality control in the rest of the batch. The hsa gmp guidelines for cells, tissues and gene therapy products (ctgtp) incorporate the requirements of the. Record keeping is key to this process. How are businesses required to control & maintain best practice throughout the life science supply chain? This paper proposes a regulatory. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.
From www.fpm.org.uk
The ethics of conducting clinical trials in the search for treatments Life Science Value Chain Must Meet Regulatory Guidelines For Gmp This paper proposes a regulatory value chain model (rvcm) that policymakers and regulators can use as a conceptual framework to guide. The hsa gmp guidelines for cells, tissues and gene therapy products (ctgtp) incorporate the requirements of the. By adhering to gmp guidelines, organisations mitigate risks, prevent contamination, and meet regulatory. How are businesses required to control & maintain best. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.
From www.linkedin.com
How to Create Value from Data to Your Business (A Product Management Life Science Value Chain Must Meet Regulatory Guidelines For Gmp Regulatory approval is the final stage in drug development, and it involves obtaining approval from regulatory agencies. This paper proposes a regulatory value chain model (rvcm) that policymakers and regulators can use as a conceptual framework to guide. Gmps must be in place to ensure quality control in the rest of the batch. Record keeping is key to this process.. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.
From old.sermitsiaq.ag
Value Chain Diagram Template Life Science Value Chain Must Meet Regulatory Guidelines For Gmp This paper proposes a regulatory value chain model (rvcm) that policymakers and regulators can use as a conceptual framework to guide. By adhering to gmp guidelines, organisations mitigate risks, prevent contamination, and meet regulatory. Gmps must be in place to ensure quality control in the rest of the batch. How are businesses required to control & maintain best practice throughout. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.
From wintongibbons.com
Framework Healthcare Value Chain Life Science Value Chain Must Meet Regulatory Guidelines For Gmp The hsa gmp guidelines for cells, tissues and gene therapy products (ctgtp) incorporate the requirements of the. This paper proposes a regulatory value chain model (rvcm) that policymakers and regulators can use as a conceptual framework to guide. How are businesses required to control & maintain best practice throughout the life science supply chain? Regulatory approval is the final stage. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.
From www.netsuite.com
What Is Value Chain? An Expert Guide NetSuite Life Science Value Chain Must Meet Regulatory Guidelines For Gmp Gmps must be in place to ensure quality control in the rest of the batch. Regulatory approval is the final stage in drug development, and it involves obtaining approval from regulatory agencies. The hsa gmp guidelines for cells, tissues and gene therapy products (ctgtp) incorporate the requirements of the. Record keeping is key to this process. By adhering to gmp. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.
From www.eficiens.com
L'assurance en effervescence N°63 26 février 2021 Eficiens Life Science Value Chain Must Meet Regulatory Guidelines For Gmp How are businesses required to control & maintain best practice throughout the life science supply chain? By adhering to gmp guidelines, organisations mitigate risks, prevent contamination, and meet regulatory. Regulatory approval is the final stage in drug development, and it involves obtaining approval from regulatory agencies. Record keeping is key to this process. The hsa gmp guidelines for cells, tissues. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.
From present5.com
Session id 40263 Oracle Life Sciences Platform and Life Science Value Chain Must Meet Regulatory Guidelines For Gmp The hsa gmp guidelines for cells, tissues and gene therapy products (ctgtp) incorporate the requirements of the. This paper proposes a regulatory value chain model (rvcm) that policymakers and regulators can use as a conceptual framework to guide. How are businesses required to control & maintain best practice throughout the life science supply chain? Record keeping is key to this. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.
From www.pinterest.com
The different steps in the biopharma supply chain Supply chain Life Science Value Chain Must Meet Regulatory Guidelines For Gmp By adhering to gmp guidelines, organisations mitigate risks, prevent contamination, and meet regulatory. Gmps must be in place to ensure quality control in the rest of the batch. Regulatory approval is the final stage in drug development, and it involves obtaining approval from regulatory agencies. How are businesses required to control & maintain best practice throughout the life science supply. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.
From dupress.deloitte.com
Global value chains Strategy or process? Deloitte Insights Life Science Value Chain Must Meet Regulatory Guidelines For Gmp Regulatory approval is the final stage in drug development, and it involves obtaining approval from regulatory agencies. How are businesses required to control & maintain best practice throughout the life science supply chain? This paper proposes a regulatory value chain model (rvcm) that policymakers and regulators can use as a conceptual framework to guide. By adhering to gmp guidelines, organisations. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.
From www.mdpi.com
Sustainability Free FullText Case Study Analysis on AgriFood Life Science Value Chain Must Meet Regulatory Guidelines For Gmp How are businesses required to control & maintain best practice throughout the life science supply chain? The hsa gmp guidelines for cells, tissues and gene therapy products (ctgtp) incorporate the requirements of the. Gmps must be in place to ensure quality control in the rest of the batch. By adhering to gmp guidelines, organisations mitigate risks, prevent contamination, and meet. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.
From www.collidu.com
Pharmaceutical Value Chain PowerPoint Presentation Slides PPT Template Life Science Value Chain Must Meet Regulatory Guidelines For Gmp Regulatory approval is the final stage in drug development, and it involves obtaining approval from regulatory agencies. Record keeping is key to this process. The hsa gmp guidelines for cells, tissues and gene therapy products (ctgtp) incorporate the requirements of the. How are businesses required to control & maintain best practice throughout the life science supply chain? This paper proposes. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.
From www.ltimindtree.com
Intelligent RPA for Life Science LTI Life Science Value Chain Must Meet Regulatory Guidelines For Gmp Record keeping is key to this process. Gmps must be in place to ensure quality control in the rest of the batch. How are businesses required to control & maintain best practice throughout the life science supply chain? The hsa gmp guidelines for cells, tissues and gene therapy products (ctgtp) incorporate the requirements of the. This paper proposes a regulatory. Life Science Value Chain Must Meet Regulatory Guidelines For Gmp.