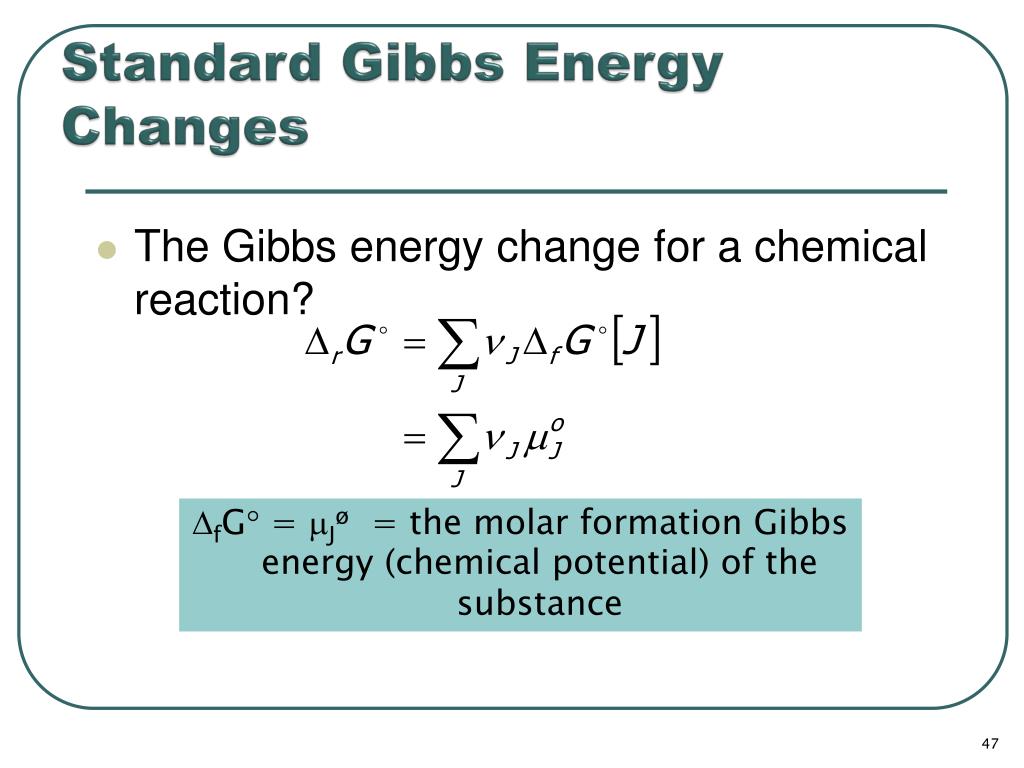
Standard Vs Non Standard Gibbs Free Energy . the change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. summary of gibbs free energy. calculate standard free energy change for a process using senthalpies of formation and the entropies for its reactants and. — the change in gibbs free energy under nonstandard conditions, δ g, can be determined from. The reaction is favourable means spontaneous if the value of δg is negative. this page introduces gibbs free energy (often just called free energy), and shows how it can be used to predict the. because all three criteria are assessing the same thing—the spontaneity of the process—it would be most surprising. the relationship between free energy and equilibrium constants. — δh° and δs° represent the change in enthalpy and entropy between product and reactant but they do not mean. When a reaction leaves the standard state because of a change in the ratio of the.
from www.slideserve.com
because all three criteria are assessing the same thing—the spontaneity of the process—it would be most surprising. — δh° and δs° represent the change in enthalpy and entropy between product and reactant but they do not mean. calculate standard free energy change for a process using senthalpies of formation and the entropies for its reactants and. this page introduces gibbs free energy (often just called free energy), and shows how it can be used to predict the. When a reaction leaves the standard state because of a change in the ratio of the. the relationship between free energy and equilibrium constants. — the change in gibbs free energy under nonstandard conditions, δ g, can be determined from. The reaction is favourable means spontaneous if the value of δg is negative. summary of gibbs free energy. the change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the.
PPT Spontaneity and Equilibrium in Chemical Systems PowerPoint
Standard Vs Non Standard Gibbs Free Energy — δh° and δs° represent the change in enthalpy and entropy between product and reactant but they do not mean. When a reaction leaves the standard state because of a change in the ratio of the. — δh° and δs° represent the change in enthalpy and entropy between product and reactant but they do not mean. the relationship between free energy and equilibrium constants. the change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. because all three criteria are assessing the same thing—the spontaneity of the process—it would be most surprising. calculate standard free energy change for a process using senthalpies of formation and the entropies for its reactants and. summary of gibbs free energy. this page introduces gibbs free energy (often just called free energy), and shows how it can be used to predict the. — the change in gibbs free energy under nonstandard conditions, δ g, can be determined from. The reaction is favourable means spontaneous if the value of δg is negative.
From www.slideserve.com
PPT Gibbs Free Energy PowerPoint Presentation, free download ID6125811 Standard Vs Non Standard Gibbs Free Energy because all three criteria are assessing the same thing—the spontaneity of the process—it would be most surprising. the relationship between free energy and equilibrium constants. The reaction is favourable means spontaneous if the value of δg is negative. — the change in gibbs free energy under nonstandard conditions, δ g, can be determined from. summary of. Standard Vs Non Standard Gibbs Free Energy.
From www.youtube.com
Standard vs NonStandard Gibbs Free Energy Graphs YouTube Standard Vs Non Standard Gibbs Free Energy because all three criteria are assessing the same thing—the spontaneity of the process—it would be most surprising. — the change in gibbs free energy under nonstandard conditions, δ g, can be determined from. calculate standard free energy change for a process using senthalpies of formation and the entropies for its reactants and. When a reaction leaves the. Standard Vs Non Standard Gibbs Free Energy.
From www.pinterest.com
Gibbs Free Energy Free energy, Chemistry lessons, Chemistry study guide Standard Vs Non Standard Gibbs Free Energy the change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. because all three criteria are assessing the same thing—the spontaneity of the process—it would be most surprising. this page introduces gibbs free energy (often just called free energy), and shows how it can be used to. Standard Vs Non Standard Gibbs Free Energy.
From www.researchgate.net
The standard Gibbs free energy and lowest reaction temperature of Standard Vs Non Standard Gibbs Free Energy summary of gibbs free energy. — δh° and δs° represent the change in enthalpy and entropy between product and reactant but they do not mean. the change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. because all three criteria are assessing the same thing—the spontaneity. Standard Vs Non Standard Gibbs Free Energy.
From www.youtube.com
Standard State Gibbs Free Energy vs NonStandard State Gibbs Free Energy Standard Vs Non Standard Gibbs Free Energy When a reaction leaves the standard state because of a change in the ratio of the. because all three criteria are assessing the same thing—the spontaneity of the process—it would be most surprising. calculate standard free energy change for a process using senthalpies of formation and the entropies for its reactants and. — the change in gibbs. Standard Vs Non Standard Gibbs Free Energy.
From byjus.com
18. Standard free gibbs energies of formation at 298K are 237.2, 394.4 Standard Vs Non Standard Gibbs Free Energy The reaction is favourable means spontaneous if the value of δg is negative. — the change in gibbs free energy under nonstandard conditions, δ g, can be determined from. — δh° and δs° represent the change in enthalpy and entropy between product and reactant but they do not mean. because all three criteria are assessing the same. Standard Vs Non Standard Gibbs Free Energy.
From quizlonesomely.z21.web.core.windows.net
Standard Free Energy Change Formula Standard Vs Non Standard Gibbs Free Energy calculate standard free energy change for a process using senthalpies of formation and the entropies for its reactants and. When a reaction leaves the standard state because of a change in the ratio of the. the relationship between free energy and equilibrium constants. The reaction is favourable means spontaneous if the value of δg is negative. summary. Standard Vs Non Standard Gibbs Free Energy.
From www.pinterest.es
(11) Gibbs free energy and spontaneity (article) Khan Academy Standard Vs Non Standard Gibbs Free Energy — the change in gibbs free energy under nonstandard conditions, δ g, can be determined from. the change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. The reaction is favourable means spontaneous if the value of δg is negative. summary of gibbs free energy. the. Standard Vs Non Standard Gibbs Free Energy.
From lavelle.chem.ucla.edu
Gibbs Free Energy in Reaction Profiles CHEMISTRY COMMUNITY Standard Vs Non Standard Gibbs Free Energy — δh° and δs° represent the change in enthalpy and entropy between product and reactant but they do not mean. this page introduces gibbs free energy (often just called free energy), and shows how it can be used to predict the. — the change in gibbs free energy under nonstandard conditions, δ g, can be determined from.. Standard Vs Non Standard Gibbs Free Energy.
From www.chegg.com
Table A1 The standard Gibbs free energy changes for Standard Vs Non Standard Gibbs Free Energy — the change in gibbs free energy under nonstandard conditions, δ g, can be determined from. The reaction is favourable means spontaneous if the value of δg is negative. summary of gibbs free energy. this page introduces gibbs free energy (often just called free energy), and shows how it can be used to predict the. the. Standard Vs Non Standard Gibbs Free Energy.
From www.slideserve.com
PPT Gibbs Free Energy PowerPoint Presentation, free download ID4493728 Standard Vs Non Standard Gibbs Free Energy — δh° and δs° represent the change in enthalpy and entropy between product and reactant but they do not mean. this page introduces gibbs free energy (often just called free energy), and shows how it can be used to predict the. — the change in gibbs free energy under nonstandard conditions, δ g, can be determined from.. Standard Vs Non Standard Gibbs Free Energy.
From mmbah.blogspot.com
Change In Gibbs Free Energy Equation Mmbah Standard Vs Non Standard Gibbs Free Energy The reaction is favourable means spontaneous if the value of δg is negative. because all three criteria are assessing the same thing—the spontaneity of the process—it would be most surprising. — δh° and δs° represent the change in enthalpy and entropy between product and reactant but they do not mean. — the change in gibbs free energy. Standard Vs Non Standard Gibbs Free Energy.
From www.slideserve.com
PPT Gibbs Free Energy PowerPoint Presentation, free download ID2198185 Standard Vs Non Standard Gibbs Free Energy — the change in gibbs free energy under nonstandard conditions, δ g, can be determined from. because all three criteria are assessing the same thing—the spontaneity of the process—it would be most surprising. the relationship between free energy and equilibrium constants. The reaction is favourable means spontaneous if the value of δg is negative. this page. Standard Vs Non Standard Gibbs Free Energy.
From www.chemistrylearner.com
Gibbs Free Energy Definition, Equation, Unit, and Example Standard Vs Non Standard Gibbs Free Energy When a reaction leaves the standard state because of a change in the ratio of the. calculate standard free energy change for a process using senthalpies of formation and the entropies for its reactants and. because all three criteria are assessing the same thing—the spontaneity of the process—it would be most surprising. this page introduces gibbs free. Standard Vs Non Standard Gibbs Free Energy.
From www.researchgate.net
Relationship between standard Gibbs free energy and temperature Standard Vs Non Standard Gibbs Free Energy summary of gibbs free energy. because all three criteria are assessing the same thing—the spontaneity of the process—it would be most surprising. — the change in gibbs free energy under nonstandard conditions, δ g, can be determined from. The reaction is favourable means spontaneous if the value of δg is negative. calculate standard free energy change. Standard Vs Non Standard Gibbs Free Energy.
From lesmylscuisine.blogspot.com
Standard Gibbs Free Energy Equation Lesmyl Scuisine Standard Vs Non Standard Gibbs Free Energy The reaction is favourable means spontaneous if the value of δg is negative. summary of gibbs free energy. the change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. When a reaction leaves the standard state because of a change in the ratio of the. calculate standard. Standard Vs Non Standard Gibbs Free Energy.
From www.slideserve.com
PPT First Law of Thermodynamics PowerPoint Presentation, free Standard Vs Non Standard Gibbs Free Energy the change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. because all three criteria are assessing the same thing—the spontaneity of the process—it would be most surprising. — the change in gibbs free energy under nonstandard conditions, δ g, can be determined from. calculate standard. Standard Vs Non Standard Gibbs Free Energy.
From www.researchgate.net
Relationship between standard Gibbs free energy and temperature Standard Vs Non Standard Gibbs Free Energy the relationship between free energy and equilibrium constants. summary of gibbs free energy. because all three criteria are assessing the same thing—the spontaneity of the process—it would be most surprising. calculate standard free energy change for a process using senthalpies of formation and the entropies for its reactants and. When a reaction leaves the standard state. Standard Vs Non Standard Gibbs Free Energy.